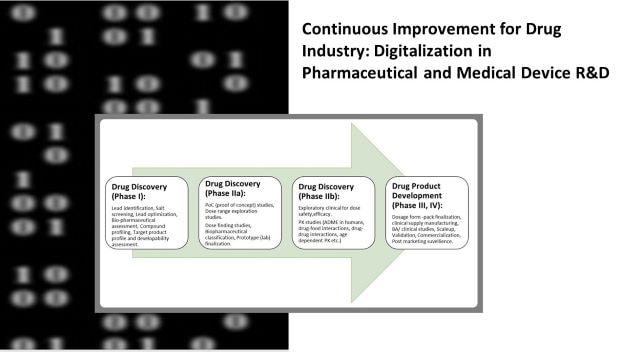
In this blogpost I shall share- Tips and Quick wins for digitalization in pharmaceutical and medical device R&D.
Pharmaceutical business has several customer touch points for example- distributors, doctors, healthcare staff, caregivers, and patients (who are the ultimate consumer). Obtaining VOC is vital in any continuous improvement campaign, same with digitalization. Install suitable mechanisms to obtain feedback via surveys, interviews etc. Note that such feedback must be gathered periodically to ensure that digitalization campaigns run smooth, error-free and rapid as planned.
Tip: Transition to digitalization is often more complex than anticipated. Some roadblocks on the digitalization transition aren’t apparent until it’s under way.
Note that, continuous improvement in pharmaceutical and medical device R&Ds can happen via 15 different pathways. Digitalization is just one such continuous improvement pathway.
Digital is a journey and organizations can anticipate both false starts and quick wins. Even being a fast follower, which by definition requires the ability to move quickly can be elusive.
Organizations with serious intentions of moving quickly could find themselves lagging behind as unexpected challenges set them back. Those that have not yet started could find themselves on the back foot when others demonstrate success.
Digital transformation in drug industry presents the opportunity to ensure better outcomes for patients via targeted therapies, significantly reduced cost of drug development and accelerated cycle times to get treatments to patients faster.
Digitization and digitalization are not something companies can debate on to have or have not. It isn’t about building a competitive advantage or a core differentiator – it is about staying relevant in business in the very near future.
To make it successful, it is less about where you start than about where you want to go. So, make sure you have a vision for going digital and that the pilots and initiatives you run tie back to it and can help it scale.
Digitalization is a continuous improvement process. Hence, organizations must keep pace with advances in digitalization technology along with advances in pharmaceutical sciences, market dynamics and more importantly patient needs.
Continuous improvement in R&D be it via digitalization or any other pathway is a top-down initiative. Leaders must make continuous improvement in pharma or medical device R&D a priority and an integral part of corporate goals.
Digital transformation can drive success in achieving key strategic R&D imperatives. However, the drive to digitalize needs to come from the C-suite or senior R&D leadership.
R&D digitalization involves big cost (although few phases of digitalization can be done on a shoe-string budget). But it is the need of the business to stay relevant and stay competitive. Hence, companies must strategically plan their digitalization journey.
Adopting a digital mindset is thus a new business imperative. A comprehensive R&D strategy is critical to enable companies to move and process large amounts of data effectively to make data-driven scientific and business decisions quickly and accurately to generate evidence in support of future product value propositions. This will require new capabilities; new skills sets and new leadership mindset.
Digitalization will be a necessity in pharma/ medical device R&Ds in years to come regardless of whether it is an innovator company or a generic player. The extent and magnitude of digitalization needed of course shall be different.
Digitalization in R&D will definitely bring a positive change in working of the R&D department, and its ripples of impact will be seen across all departments within the organization. Therefore, it is crucial that this digital transformation is smooth, does not adversely impact the top line and bottom line while the change is happening.
The best way to begin digitalization initiative is to start with Kaizen.
Kaizen is a time-tested Japanese technique of business transformation and change management. The spirit of Kaizen involves small changes done continuously over time to reap exponential rewards.
Organizations could do Kaizen-ing to build digital strategy as well; but Kaizen certainly must be used during the digital strategy execution phase. You can know more about Kaizen for Pharmaceuticals and medical devices industry here.
Conduct multiple Kaizen events to navigate your digital transformation journey successfully.
The future of pharmaceutical R&D ten years ahead as I foresee is an entirely new panorama: a world where drug discovery is driven by machine learning and advanced analytics mining large data sets, enabling us to understand and visualize interaction with targets and to predict in silico a molecule’s likelihood of success, meet approval and reach market.
Done right digitalization, the size of the opportunity could be $50–150 billion of EBITDA across the industry.
Note that there are fifteen different Continuous Improvement pathways for pharmaceutical and medical device R&Ds. Are you planning a Continuous Improvement initiative for your R&D division?
Related reading:
#continuousimprovement #digitization #digitalization #kaizenforpharmaceuticals #digitaltransformation #digitalizationinpharmaceuticalindustry #kaizenindigtaltransformation