Continuous improvement can transform businesses from being good to great or turnaround a sick business to become a profit-making enterprise. If practiced correctly continuous improvement brings-in exponential benefits to the company, its employees and customers ( i.e. patients).
When I say continuous improvement for pharmaceutical companies, improvement of R&Ds is a major aspect- be it- R&D design, functioning or output.
An effective productive R&D can develop products fast and give first-to-file benefit. If first-to-file is your objective then make sure your R&D labs have installed a culture of continuous improvement to build-in capable infrastructure, efficient & effective business processes and a talent pool having relevant skill sets.
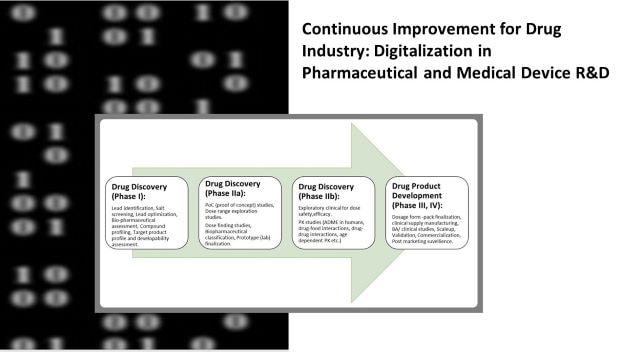
There are 15 different continuous improvement pathways for pharmaceutical and medical device R&Ds; I shall discuss one such pathway- Digital transformation in Pharmaceutical and Medical Device R&Ds.
Digital transformation involves- Digitization and digitalization. Both digitization and digitalization are two significant tools for improving R&D functioning and output.
Digitization is conversion of data and processes from analog to digital whereas, digitalization embraces the ability of digital technology to collect data, establish trends, make better business decisions, in toto transform ‘how work happens’.
In this nine-part blogpost series, I shall touch upon digitalization in pharmaceutical and medical device R&D, benefits of digitalization & challenges to overcome, how to select the right digitalization platform, building blocks for digital transformation, success metrics and how to transit from existing R&D setup to a digitalized R&D.
In Part 2 of this series, I shall discuss on- Why the need for digitalization in pharma and medical device R&D labs?
Meanwhile, note that there are fifteen different Continuous Improvement pathways for pharmaceutical and medical device R&Ds. Are you planning a Continuous Improvement initiative for your R&D division?
Upcoming blogposts in this series-
Part 3: How to go for digitalization in R&D.
Part 4: Advantages of digitalization in pharma and medical device R&Ds.
Part 5: Digitalization in various phases of drug research and development.
Part 6: Selecting the right R&D digitalization platform.
Part 7: Six building blocks for digital transformation in pharmaceutical and medical device R&Ds.
Part 8: How to transform existing R&D setup to a digitalized R&D.
Part 9: Tips and Quick wins of digitalization in pharmaceutical and medical device R&D.
Related reading:
#businessprocessimprovement #continuousimprovement #digitalization #digitalizationinpharmaceuticalindustry