Considering the vast benefits of JIT methodology, I recommended using it in R&D division of a Life science company (my client). This company had a major set back; consequently, had a severe cash-crunch and was looking for a better option to sustain business and avoid employee layoffs.
This project was accomplished few years ago. However, the learnings are relevant today perhaps more than ever before.
The reason being that the Covid-19 pandemic has created a funds-crunch in companies big and small; though start-ups, small and mid-size organizations bear more brunt. Innovation portfolios are being driven in the slow lane, R&D budgets are getting axed and yet the divisions must do more with 'still' less. |
Some of commonly used raw materials in life science R&Ds include- Active ingredients (such as bulk drug, herbal extract), additives (such as bulking agents, colors, flavors, perfumes, emulsifiers, solvents etc.), packing materials (such as tubes, bottles, caps, wads, aluminum foil, paper, glue), machine parts (viz. tablet punches, dies, chromatography columns).
These consumable items are extensively used during experimentation for new product development and testing. In fact, the R&D department cannot function at all if consumables are not provided.
In research-based life science companies and contract research organizations, consumables comprise of 20 – 45 % of their R&D budget. Therefore, any savings in consumable expenses help organizations to lower over-head costs big-time, and Just-in-time methodology does just that!
A four-step approach was taken for this project-
Step 1: The existing supply chain process was mapped, inventory demand-supply schedules reviewed and gaps in these processes were identified.
The objective was to re-design the supply chain process to include Just-in-time technique to lower R&D over-heads (without disturbing R&D output) and the savings generated thereof could be utilized for other needs of the business.
Step 2: A supply chain process improvement strategy was designed.
Note that, there are several dos and don’ts prior to applying Just-in-time technique for process improvement or process design. One of such important to-dos, is to ensure all items being sourced comply to CQA (Critical Quality Attributes).
Step 3: A core team comprising heads of R&D, Manufacturing, Quality, Regulatory and Procurement was formed.
Then all consumable raw materials were classified into- expensive, moderate and cheap items, based on their cost and availability. Vendors were approached to provide samples (of raw materials) for testing. Once test results proved compliance to CQA, the vendors were short-listed.
Step 4: Just-in-time process was set up.
This also involved effective collaboration between R&D, resource planning and quality groups. Then, meetings were conducted with vendors to provide raw materials at negotiated price to R&D. Vendors for expensive raw materials were approached first, followed by those for moderate and cheaper items.
Note that-
- Depending on the size of the organization and the number of consumable items to be worked on, it might take from 2 to 8 weeks to fully set up Just-in-time in a company.
- Just-in-time methodology encourages sourcing from local or regional vendors rather than overseas. Should a company go for an overseas vendor, then make sure that vendor has a distribution warehouse situated locally or regionally.
- Savings from Just-in-time implementation are further augmented when R&D's follow Quality-by-Design (QbD) approach for product development.
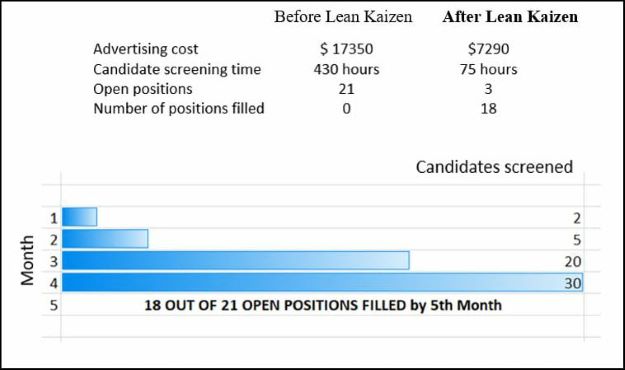
Result Dashboard:
- Through flawless execution of Just-in-time technique, around 35% of R&D over-head cost was saved in the first year.
- Besides lowering over-heads, Just-in-time also helped free-up warehouse capacities.
- Continued implementation of Just-in-time technique maximized over-head savings up to around 80%.
#JustInTime #ImproveOperatingEfficiency #ContinueInnovation #ContinueProductDevelopmentInPandemic #Covid19 #ImproveOperatingExcellence #ProcessImprovement #ProcessDesign