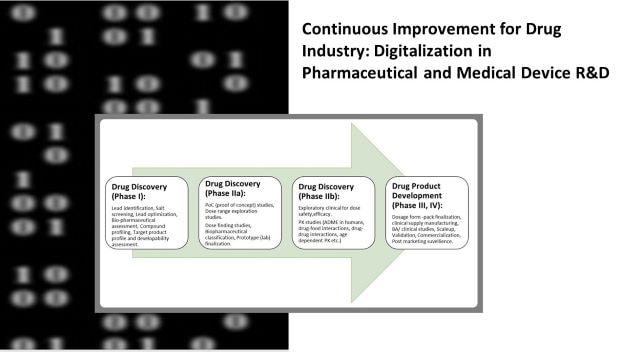
In this blogpost, I shall highlight some of the salient advantages of digitalization in pharma and medical device R&Ds.
Advantages of Digitalization in Pharmaceutical and Medical Device R&D
Artificial intelligence (AI), advanced analytics and automation are changing ways of working in scientific research. The umbrella category of AI and advanced analytics of digital technology has the maximum potential to improve R&D productivity.
Close behind are identification of biomarkers, use of EHRs (Electronic Health Records) and Clinical Registries.
A significant advantage of digitalization in drug development is creation of targeting biomarkers as the therapeutic approach, followed closely by gene therapies and customized therapies targeting specific disease stages & comorbidities.
Digitalization opens pharma R&D horizons in areas such as genomics which further leads to breakthroughs in precision medicine. It also creates opportunities for decentralized clinical trials, unleashing future innovation in digi-ceuticals and healthcare wearables.
In the allied chemicals sector too, R&D digitalization facilitates designing and modeling new materials and composites. Digitalized chemical innovation is the foundation for everyday products from smartphones and fiberoptic network cables to autonomous vehicles. Digitalization enables researchers to better predict the properties of a material, the performance of an active molecule or its toxicological effect.
As quantum computing- an element of digitalization, allows deeper molecular modeling, medical device research and development specialists expect quantum computing to open new material prospects in specialties such as batteries, semiconductors, superconductors, composites and opto-electronic gadgets.
Further, quantum computing-based molecular modelling helps researchers to identify the most effective molecular designs or structures before having to synthesize molecules in laboratory settings. AI helps glean the best compound in the early stage, so researchers can skip over the very long discovery processes. For example- using AI to select or optimize the antibodies and evaluate efficacy or toxicity in the preclinical stage.
Digitalization explores the space of interest for discovery, guides researchers to do more focused experiments and generate data to refine the experimentation process further.
As therapies are designed as targeted dosage forms, the efforts to identify those patients who may benefit most from a drug takes considerable amount of time and effort. Digitalization helps these efforts by leveraging digital technology to help find the right people set for the right trials.
For example- AI improves patient-trial matching by crunching large datasets like electronic health records, genomic data, medical literature, trial databases, inadequate enrollment of patients in trials—too small a sample, too high a drop-off rate, or suboptimal cohort selection. Failing to exclude individuals using another medicine that could interfere with the tested one—is a major cause of trial failure.
Once underway, advanced analytics can enable adaptive clinical trials, where researchers modify a live trial, such as by refining the sample size, changing the allocation of patients to ‘control’ versus ‘trial’ arms, and stopping a trial early based on likely success or failure.
A significant value driver within clinical R&D is reinventing how companies engage physicians, patients, and investigators at a granular level. Through digitalization, the medical affairs teams can understand the requirements of individual physicians (as well as other stakeholders) and to deliver precise information on-demand.
Lowering the costs of trials and improving experimental data are worthy ends in themselves, but companies can also use digital technology to identify new lines of therapy.
For instance, in health care, the quality of data available to researchers helps in the development of therapeutic areas like immunotherapy, which alters the human immune system to better fight diseases like cancer. This is a far cry from current therapies like radiotherapy and chemotherapy which bring debilitating side effects and are often palliative rather than curative. Digital technologies like wearables, implants, and digestible can show how a therapy is working in richer detail via digital biomarkers.
An overarching benefit of digitalization in pharma R&D is improving the quality of new products before they enter the experiment and clinical trial process. Digital innovation can improve the quality of experiments from optimizing design and protocols to generating better data about the drug candidate’s effectiveness.
Big data and AI enabled modelling processes allow researchers to move from static processes to more fluid and adaptable working procedures for drug discovery and R&D pipeline portfolio management.
Moreover, analytics, simulation, prediction, and modeling show researchers more about how an innovation might fare in the real world. This eliminates drug candidates that might once have incurred costs in a failed pilot or pivotal trial due to shortcomings that researchers were unable to spot in an earlier phase.
Further, in a digitalized formulation R&D, with super imposable information from recipe builder on bio-chemical properties of active(s) and additives, enforced patent and regulatory data, with a click of a mouse key, formulators can checkout series of formulation suggestions. These formulation suggestions can be further evaluated by digital experimentation.
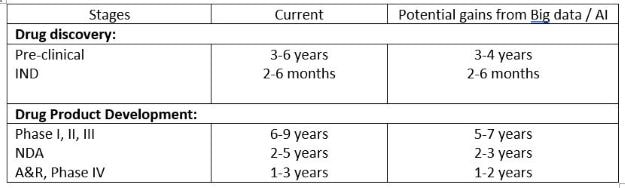
On an average, post digitalization in drug development, the time from drug discovery thru product commercialization can be decreased by as much as 6 years!
In the next part of this series, I shall elaborate on- Digitalization in various phases of drug research and development.
Meanwhile, note that there are fifteen different Continuous Improvement pathways for pharmaceutical and medical device R&Ds. Are you planning a Continuous Improvement initiative for your R&D division?
Upcoming blogposts in this series-
Part 6: Selecting the right R&D digitalization platform.
Part 7: Six building blocks for digital transformation in pharmaceutical and medical device R&Ds.
Part 8: How to transform existing R&D setup to a digitalized R&D.
Part 9: Tips and Quick wins of digitalization in pharmaceutical and medical device R&D.
Related reading:
#businessprocessimprovement #continuousimprovement #digitalization #digitization #digitaltransformation #digitaltransformationinR&D #digitalizationinR&D #continuousimprovementinR&D #advantagesofdigitalization