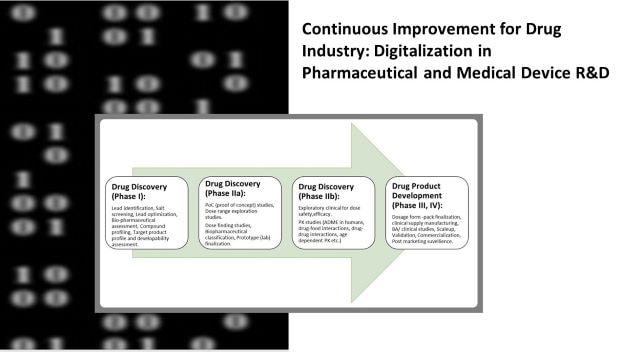
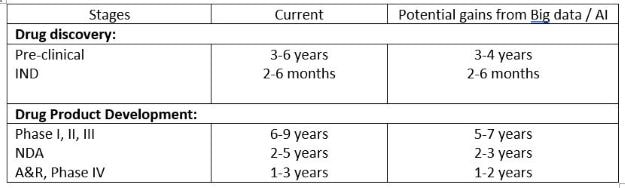
Then, companies must understand the regulations, standards, and costs of quality. Without this information, they will not be able to effectively apply Lean manufacturing and operational excellence principles. But how do we get there? This post looks at three key aspects of medical device manufacturing, and how they can be applied to the entire sector.
As the industry continues to undergo unprecedented regulatory changes, mergers and divestitures, and value-based payment models, companies must learn how to improve operational performance. To cope with these challenges, companies apply technology and take advantage of outside managed service partners to handle these tasks. These partnerships offer companies the advantage of freeing up internal resources to focus on core aspects of the business. It is essential to find a partner that understands the medical devices industry and has experience in the field.
Lean in Ireland has led to massive success and has turned the country into a global hub for MedTech. The growing scrutiny from different global regulatory authorities has also increased uptake of organizational excellence. This is because companies are constantly monitoring regulatory bodies in different jurisdictions. However, fear of upsetting regulatory authorities has often slowed down the implementation of change in manufacturing. If organizations don't embrace these changes, they risk losing their customers.
Lean is not a new concept, but the medical devices industry needs to look at it in a different way. Although Lean is commonly associated with the automotive industry, it has been slowly adapted to the highly regulated medical devices industry. The benefits of implementing Lean methods in a highly regulated environment are numerous. For one, Lean is not just about eliminating waste. Lean is about enhanced quality, process capability, process reliability, business efficiency and profitability!
For more about operational excellence, checkout Top Ten Strategic Decision-Making Tools for Operational Excellence
Related Reading:
- Kaizen for pharmaceutical, medical device and biotech industries
- How to cut costs strategically using Kaizen
- Streamline processes and workflows with Gemba Walk.
- Top Ten Strategic Decision-Making Tools for Operational Excellence
Follow Shruti on Twitter, Facebook, YouTube, LinkedIn
Categories: Lifesciences | Operational Excellence
Keywords and Tags:
#operationalexcellence #operationalexcellenceformedicaldeviceindustry #strategicdecisionmaking #strategy #strategymanagement #decisiontools #decisionmaking #strategicplanning #challengesofdecisionmaking