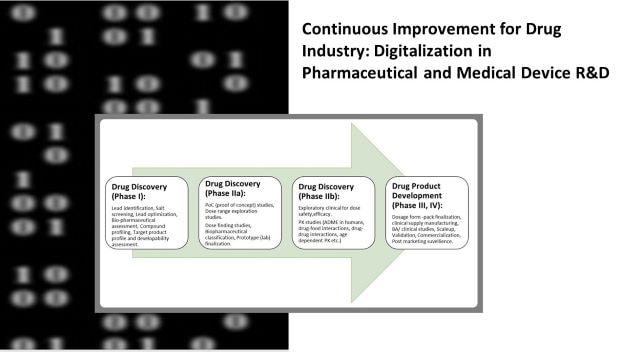
In this blogpost series I shall discuss on- Selecting the right R&D digitalization platform.
Selecting the right R&D digitalization platform-
- Does your company conduct drug discovery research?
- Does it develop innovative products or generics?
- Which therapeutic areas are considered for product development?
- Does your product pipeline entail drugs for humans or veterinary?
- Does it use advance data science currently or is all work done only in analog mode?
- How much of information must be shared with other technical/ non-technical departments within the organization?
R&D digitalization surely involves a huge cost. But it is the need of business to stay relevant and stay competitive. Hence companies must strategically plan its digitalization journey.
Continuous improvement in R&D via digitalization or any other pathway is a top-down initiative. Leaders must make 'continuous improvement' in pharma R&D a priority and an integral part of corporate goals.
There are scores of R&D digitalization platforms out there. Selecting a platform which is right for your company is key. So, what are some of the salient features to note while choosing R&D digitalization platform?
6 Salient features to note while choosing R&D digitalization platform-
2. Compliance to regulations:
Compliance to 21 CFR Part 11, ICH and all regulations impacting your product, R&D management and line of business. Also, compliance to testing, labelling and/or other product-specific regulations for drug/ drug product.
3. Product pipeline:
Developing now and what is the company aiming for? What are the types of formulations a company wants to offer in their product mix? Is it a synthetic small molecule, herbal, peptide or some other biological product, vaccine, medical device, diagnostic etc.?
Is it multiple types of dosage forms? Because a single digitalization platform should be able to cater to all needs. Which markets are you developing the products for? Because the digitalization platform must build products to comply with all regulations (concerning development, testing, labeling and other product-specific guidelines) for each market.
4. Talent building:
Talent building via employee re-skilling and/or recruitment. A bottom-up approach to changing skills and mindsets. Make Agile work in a practical way. Build capabilities and team-member engagement.
5. Facilitates remote working and/or working in Cloud.
6. Compatibility and inter operability of the platform with existing digital ecosystem within the organization:
Compatibility and inter operability of the platform with existing digital ecosystem within the company such as LIMS, ERP supply chain software, BMS (building maintenance software), equipment process automation software etc.
Compatibility and inter operability of the platform with customer engagement software channel with healthcare professionals: The med tech industry is shifting from a model that revolved around sales rep to an omni-channel world, in which HCPs (Health care professionals) are able to access information as and when they need. Although reps remain the main point of contact, companies are augmenting this relationship with new resources & tools and coordinating them across channels to address stakeholder’s needs.
Compatibility with digital marketing, portals and e-commerce platforms:
According to a published statistic, 80% of med tech companies shifted more than 20% of their marketing expenditures to digital channels in 2020. Further, 2/3rds of med tech companies expect online channels to account for more than 20% of their revenue by 2025.
Omni-channel engagement is not about making tweaks to existing models. It is about shifting to a new model in which Agile teams bring design thinking, digital engagement and analytics to help sales rep deliver the right message at the right time through the right channel to the right stakeholder.
In the next part of this series, I shall discuss on- Six building blocks for digital transformation in pharmaceutical and medical devices R&D.
Meanwhile, note that there are fifteen different Continuous Improvement pathways for pharmaceutical and medical device R&Ds. Are you planning a Continuous Improvement initiative for your R&D division?
Upcoming articles in this series-
Part 8: How to transform existing R&D setup to a digitalized R&D.
Part 9: Tips and Quick wins of digitalization in pharmaceutical and medical device R&D.
Related reading-
#businessprocessimprovement #continuousimprovement #digitalization #digitalizationinpharmaceuticalindustry #selectingdigitalizationplatform #digitalizationplatform