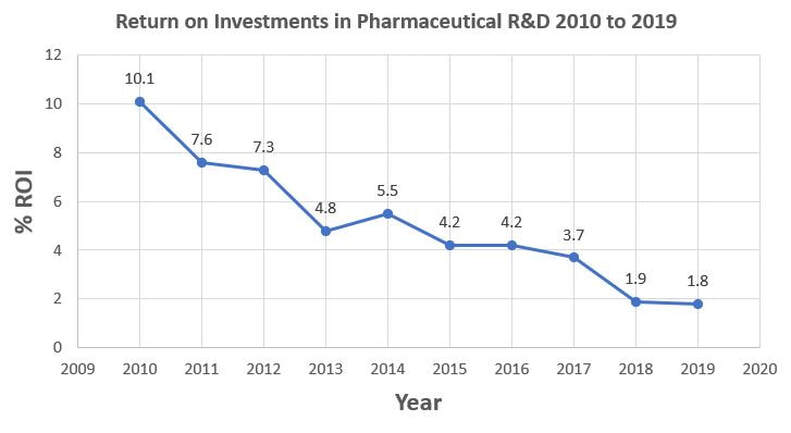
This data needs a serious investigation into- How to arrest the ROI drop and more importantly, how to increase pharmaceutical R&D ROI?
A root cause analysis of this problem could show one or many cause(s), for example- low R&D productivity, poor infrastructure, ineffective workflows, inefficient processes, poor decision-making, increased number of generic players, time taken for drug discovery thru product launch in market is higher than planned, higher number of clinical trial failures, changing patient needs, etc.
Regardless of the cause(s) of the problem(s), a sure way to increase ROI is to introduce a culture of continuous improvement in the R&D department later expanding across the entire organization.
There are more than eighteen scientific and proven continuous improvement methodologies which can be implemented in pharma R&Ds and across the organization. Some of the popular ones being Lean Six Sigma, ISO, TRIZ, Hoshin, Kaizen etc. You can know more about different continuous improvement methodologies here.
Note that continuous improvement initiatives must be customized for each company in order for it to be effective. So, a natural question that pops up is- How to start continuous improvement campaign at the R&D workplace? This is a vast topic and can run into several pages, but I shall touch upon it briefly here.
How to start Continuous Improvement campaign in pharma R&D?
Continuous Improvement initiatives must be customized for every company. A continuous improvement technique found successful at company A may not produce equally good results at company B, despite the two companies belonging to the same industry vertical.
The choice of continuous improvement methodology depends on the organizational size, culture, set of problems, product mix, financial status etc. You can know more about how to choose a continuous improvement methodology for your organization here.
Here are 9 easy steps to follow in order to initiate continuous improvement at your R&D site-
- Customize ROI data for your organization- Plot a graph of return on your R&D investments for the past decade.
- Map processes. Build Swimlanes.
- Build strategy road map for your continuous improvement campaign. Identify teams and key players.
- Conduct a Root Cause Analysis of the ROI data and R&D processes to identify root cause(s) of the problem.
- Run Kaizen Events to find potential solutions.
- Apply solutions and monitor results.
- Standardize processes.
- Improve continuously.
- Celebrate success!
Tip: Use appropriate continuous improvement tools judiciously at each of the above steps to take smart decisions from time to time.
Note that after strategy, a significant success with continuous improvement campaigns is achieved by using the right tool at the right time. There are over hundred proven Continuous Improvement tools to work with. You can check out more on Continuous Improvement tools here.
A PDCA (Plan-Do-Check-Act) cycle is extremely helpful to ensure rapid success with continuous improvement initiatives.
A common question I get while leading continuous improvement projects is- Our company is ISO certified. Do we still need to go for Kaizen?
And my response to that query is- Yes definitely. The reason being ISO methodology focuses on ‘control of processes’ underlying quality and compliance to standards. While Kaizen focuses on ‘improvement of processes’ not just quality processes but ALL processes including technical and business processes.
Having ISO and not running Kaizen along with it is like eating only a part of the pie. In fact, I would say, in order to get the maximum benefits from ISO certification, practice Kaizen.
Just 15 minutes of daily Kaizen brings stupendous rewards. I have a separate blogpost describing difference between ISO and Kaizen. You may want to check it out here.
Kaizen is a Japanese technique of process improvement. Kaizen implementation does not need big budgets and also it is super effective. You can check more about Kaizen for pharmaceutical, medical device and biotech industries here.
Are you planning to start a culture of Continuous Improvement for your workplace?
#businessprocessimprovement #iso #processefficiency #businessprocessmodification #processimprovement #continuousimprovement #kaizenforpharmaceuticalR&D #continuousimprovementforpharmaceuticalindustry #kaizen #lifesciences