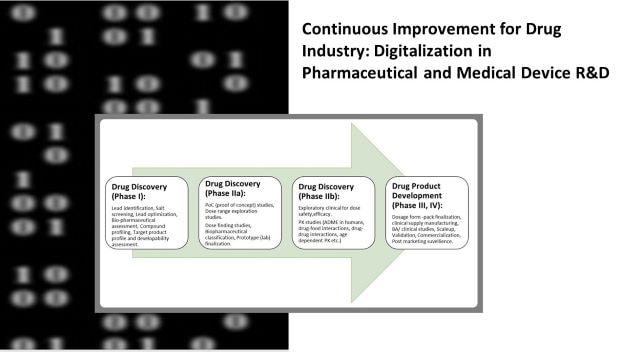
In this blogpost, I shall elaborate on- Digitalization in various phases of drug research and development.
Experiments and clinical trials carry a huge cost for drug industries, both financially and in terms of human and scientific resources. Advanced simulation, modelling, AI-based analytics, and quantum computing help identify the strongest candidate for new therapies by allowing only the most promising moieties to proceed to the costly experimental phase.
Let’s see how does digitalization impact various phases of drug development…
Digitalization in drug discovery-
Using digitalization at in silico discovery for target identification maximizes the value of data. The efficient data interoperability implies a clear data strategy, data governance and data management which shrinks drug discovery timelines significantly by 3-5 years! Also, this reduces cost of drug discovery by several million USD.
Further, besides researchers, peers and colleagues from project decision-making to portfolio management & risk optimization functions work more closely together and feel more empowered through increased data visibility and decision transparency.
Digitalization in drug product development-
Just as drug discovery, digitalization impacts how drug products are developed such as-
1. (Drug) Salt- finding study.
2. Dose-finding study.
3. Identifying ADME (Absorption Distribution Metabolism Elimination) of the drug.
4. Route-finding study-
Route finding study identifies the best way to administer the drug or whether the drug can be administered via multiple routes? Should the drug be administered only by per oral route or can it be administered via other mucosal routes such as nasal, inhalation, ophthalmic, parenteral, transdermal etc.
5. Proof of concept study/ Formulation development & analytical testing-
Digitalization allows machine learning algorithms to review raw material properties such as- drug molecule chemistry, excipient chemistry, powder dynamics, rheology, ADME , patent and regulatory data etc. to build formulation recipes and predict results. Digitalization uses Big Data to suggest formulation compositions.
For instance- in case of targeted drug delivery systems, the choice of carrier is vital. And researchers conduct studies to identify if the carrier-mediated drug transport additive should be- Niosomes, liposomes, methacrylates, ion-exchange resins, cyclodextrins, fullerenes or nanotech-buckyballs, RBC (red blood cells) or something else.
Another customary study is to find out if the formulation requires a therapeutic platform for delivery.
Needless to say, huge number of experiments get done to arrive at the ‘best’ carrier for drug delivery. Obviously, this involves huge R&D cost and time which can be saved by digitalization in product development.
Moreover, data visualization helps researchers to identify the product with best profile. Both in vitro and in vivo data can be visualized. Researchers can skip pilot BE studies if a product shows superimposable data graphics with RLD (reference listed drug). Imagine the costs saved!
Additionally, quantum computing also immensely helps product development via 505 (b) (2) pathway.
Hence, I would say if a company aims to develop products via 505 (b)(2) pathway then digitalization is the way to go. For such companies, digitalization shall save millions of dollars of R&D budget and in addition provides first-mover advantage.
Digitalization in clinical research-
AI has many potential applications in clinical trials both near- and long-term. These range from automating routine study data entry functions, to analyzing electronic health record data to find suitable candidates & clinical trial sites, to monitoring and encouraging patient compliance with study protocols, to adaptive dose finding, to discovering and modelling potential new molecules and therapies.
Big Data and real-world data (RWD) are becoming increasingly prevalent as digital devices become more adept at capturing myriad signals from patients in a variety of settings.
For example, combining historical information from EHRs (electronic data records) with imaging, genetic and molecular test data is driving the development of highly targeted oncology treatments, such as CAR-T and other cell therapies, giving hope to patients resistant to more conventional treatment approaches .
If Big Data is the raw material of digital transformation, then AI is the engine that sponsors rely on.
AI powered capabilities viz pattern recognition, evolutionary modelling etc. are essential to gather, normalize, analyze and harness large data sets that are fundamental to modern therapeutic development. Also, these models are especially useful for conducting smaller studies that are gaining importance in targeted smaller patient populations.
Additionally, applying advanced statistical models to the new data also improves pharma R&D productivity.
One of the main hurdles in clinical operations is the disconnect between clinical development plans and clinical trial protocols. In other words, between strategy and the operations.
Digitalization defines the ideal trial avatar and guides researchers on patient-centric clinical development, feasibility of the study to help them better plan the duration of recruitment and trial length, collaborate with patients in the research process, ease patient access, reduce the burden of trial participation and transform patientcare during clinical trials.
Such gained efficiencies, optimize time and cost, expedite patient enrolments, improve retention & increase study diversity, improve investigator productivity, reduce manual effort on repetitive tasks and transform patientcare during a clinical trial by paper-less tracking.
A published case study (Reference- https://www2.deloitte.com/us/en/blog/health-care-blog/2018/how-a-digital-rd-strategy-can-improve-clinical-trial-innovation-and-execution.html) indicated how digital transformation of a standard neurological test reduced clinician burden- in neurological assessment, a commonly used symbol-digit modalities test asks a participant to match basic numerals to geometric shapes according to a reference key. The lower the number of correct connections within the allotted time, the higher the level of cognitive impairment. A biopharma company made this test available to patients on an iPad at the point of care and fed test results directly into the patient’s EHRs. This eliminated the need for clinicians to spend time on conducting the test and entering data and also enabled more standardized test administration.
From understanding disease onset and progression to identifying early safety signals or food and drug interactions to engaging with patients for clinical trial participation and awareness to virtual augmented reality to conduct virtual clinical trials, digitalization enables researchers to better predict the properties of a material, the performance of an active molecule and/ or its toxicological effect.
In addition, digitalization improves efficiency of post-marketing pharmacovigilance and patient safety.
In fact, from now on in the coming decade, adopting digital technology at scale viz patient reported outcomes captured using mobile devices, digital assistants and voice recognition to virtual augmented reality (AR) to conduct virtual clinical trials and digital biomarkers as primary endpoints might just be the norm!
Digitalization will be a necessity in pharma R&Ds in years to come regardless of whether it is an innovator company or a generic player. The extent and magnitude of digitalization needed of course shall be different for the two.
Pharma, medical device R&D digitalization surely involves a huge cost. But it is the need of business to stay relevant and stay competitive. Hence companies must strategically plan its digitalization journey. One of the critical aspects of R&D digitalization is selecting the right digitalization platform.
Coming up in next part of this series- Selecting the right R&D digitalization platform!
Meanwhile, note that there are fifteen different Continuous Improvement pathways for pharmaceutical and medical device R&Ds. Are you planning a Continuous Improvement initiative for your R&D division?
Upcoming blogposts in this series-
Part 7: Six building blocks for digital transformation in pharmaceutical and medical devices R&Ds.
Part 8: How to transform existing R&D setup to a digitalized R&D.
Part 9: Tips and Quick wins of digitalization in pharmaceutical and medical device R&D.
Related reading-
#continuousimprovement #digitaltransformation #digitalization #digitalizationinpharmaceuticalindustry #digitalindrugdiscovery #digitalizationindrugproductdevelopment #digitalizationinclinicalresearch