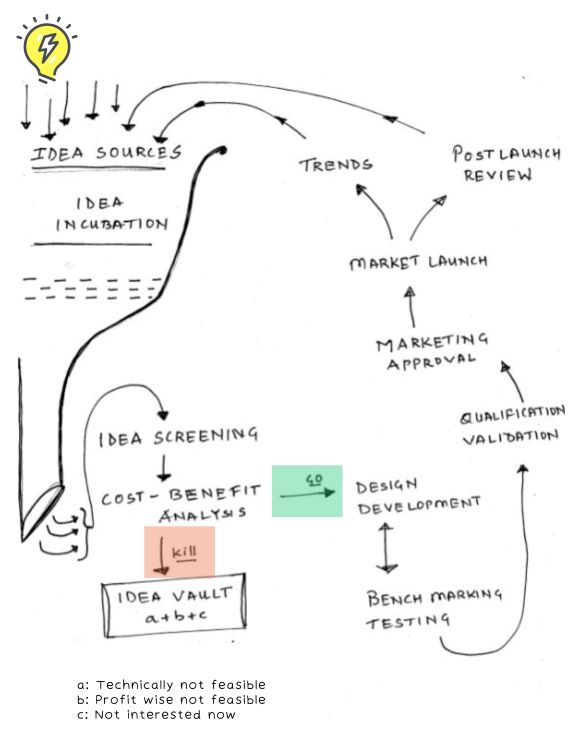
- Company’s definition of a new product.
- Portfolio project management technique employed.
- Product development pathway.
- Marketing and Sales process's abilities to gain market share.
Part 1 of this article covered pillar 1- Company’s definition of a new product, Part 2 covered pillar 2- Portfolio project management technique employed, while Part 3 covered pillar 3- Product development pathways.
In this part, I shall elaborate on pillar 4- Marketing and Sales process's ability to gain market share.
The fourth important parameter of building a profitable portfolio is introspecting and assessing your marketing and sales process(es) efficiency and effectiveness.
Start by asking questions such as-
- Where is sales growth coming from?
- Are sales happening from new products or from new markets or are they happening from growth in existing markets or increase in market share?
- How do you want your product portfolio to look like in coming 3 to 5 years?
- What is your historical level of R&D spending as a percentage of sales?
Answers to these questions will throw light on your organization’s operational capacity and capability to develop and sell new products.
Also, it is vital to establish which products must be developed for self-commercialization and which should be licensed out on fee-for-service basis. Doing this will amplify portfolio’s productivity and profitability.
Further, modifying all underlying business processes involved in the sales & marketing of products, by using suitable process improvement techniques will maximize company’s revenue. For example- A struggling pharma company increased 30% of its market share in mere 3 months by implementing Kaizen to its sales and distribution process. Know more about Kaizen for pharmaceuticals here.
There are more than eighteen scientific and effective business process improvement methodologies to choose from.
For instance, using Lean Kaizen or Agile Kaizen in improvements of processes underlying sales, marketing and distributions functions is a sure and effective way to get fastest and highest ROI.
Lean identifies waste and hence improves profit figures. Agile increases quality & speed of product development, while Kaizen improves process efficiency & effectiveness and increases rate of returns.
To ensure portfolios are managed to give best rewards, it is vital to prune it regularly. Further advanced analytics may be used to identify optimal combinations of pruning candidates.
Conclusion:
Applying Lean, Kaizen, Lean Six Sigma and other process improvement techniques to technical and business processes (regardless of industry sector) brings about successful business transformation. And applying Kaizen to sales, marketing and distribution processes augments increase in revenue.
Moreover, all three product development pathways that is Incremental change, Me-too and Disruptive development pathways are beneficial for digital products as well as the physical products such as toys, cosmetics, non-medical machinery, chemicals, over-the-counter pharmaceuticals, herbal supplements, consumer health products, telecom, healthcare etc.
Equally important is to identify right product candidates from the product mix to develop the line extensions for.
Driving process improvement to create incremental changes along the value stream increases market share of existing products. For example, Hoshin integrated with Kaizen may be used for product development processes to improve product features. As Hoshin encourages voice of customer, it brings about customer-centric product development.
In fact, one market research study revealed that the built-in kitchen traditional RVs (Recreational Vehicle) provided were going unused. Instead, RV owners relied on outdoor grills. The company therefore developed a slide-out kitchen that was a hit with customers and increased RV sales.
With cashflow drying up due to the challenges posed by the pandemic, companies more than ever before need to re-balance their product portfolios and increase efficiency of underlying processes to create value to customer, transform businesses, accelerate growth and generate higher revenues for the organization.
And, now is the time to get started …
Related reading:
- How to transform businesses via portfolio management- Part 1
- How to transform businesses via portfolio management - Part 2
- How to transform businesses via portfolio management- Part 3
- Streamline processes and workflows with Gemba Walk
- Leadership Kaizen: How can leaders keep employees happy?
#businesstransformation #businessturnaround #kaizen #lean #agile #hoshin #portfoliomanagement #continuousimprovement #businessprocessimprovement #processreengineering #processimprovement #processefficiencyimprovement #processeffectiveness #transformingorganizations #transformingbusinesses #newproductportfolio #howtodobusinesstransformation #howtoimprovebusinessefficiency #productdevelopment